
Biomark to Expand Treatment Response Trial to Advanced Lung Cancer Patient Receiving Immunotherapy
Vancouver, British Columbia – (June 29th, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:
The latest information about BioMark Diagnostics to keep you up to date.


BioMark Diagnostics is leading the world in early-stage cancer detection with an oncology focus designed to improve patient outcomes.

Vancouver, British Columbia – (June 29th, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:

Vancouver, British Columbia – (June 1st, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:

Vancouver, Colombie-Britannique – (1er juin 2021) – BioMark Diagnostics Inc. («BioMark») (CSE: BUX) (FSE: 20B)

Vancouver, British Columbia – (May 31, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:

Vancouver, British Columbia – (April 29, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:

Vancouver, British Columbia – (April 21, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:
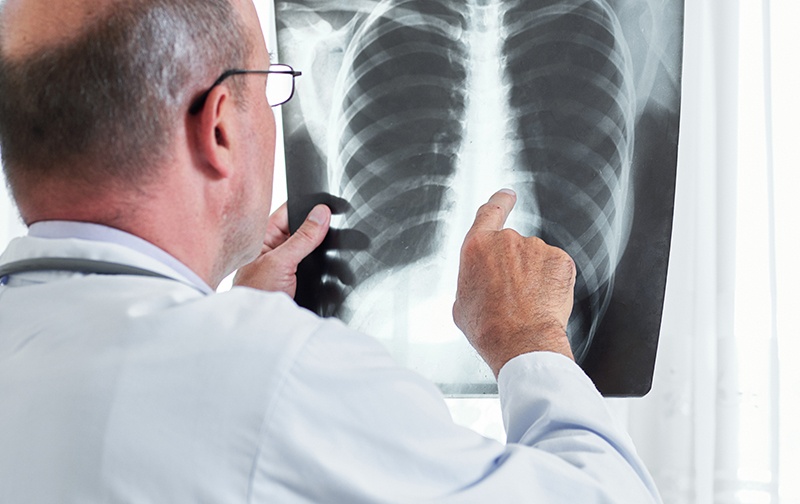
Vancouver, British Columbia – (March 16, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:

Vancouver, British Columbia – (March 2, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:
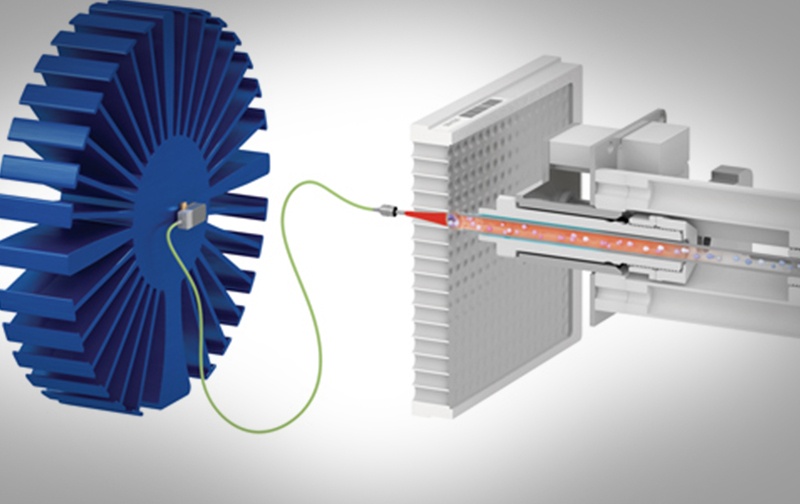
Vancouver, British Columbia – (February 16, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE:
No paperwork, no hassle. We keep things digital, easy and regular.
*Zero Spam Policy. You will need to confirm your email address to receive our news to your inbox.


1 604 370 0779
130 3851 Shell Road
Richmond BC
Canada V6X 2W2
Rashid A. Bux (CEO)
[email protected]