The Science of Metabolomics
BioMark's Early Stage Cancer Detection Technology
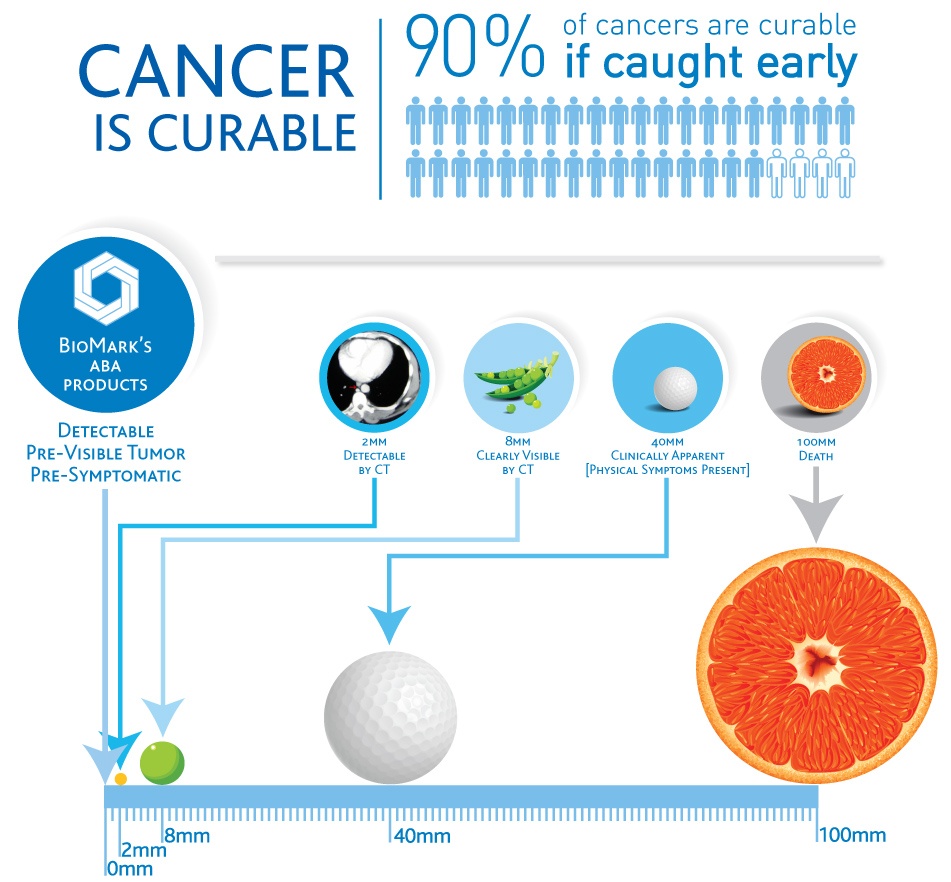
We investigate the role metabolomics plays in early stage cancer detection. The science of metabolomics is the foundation of BioMark’s research. Our goal is to improve patient comfort and outcomes.
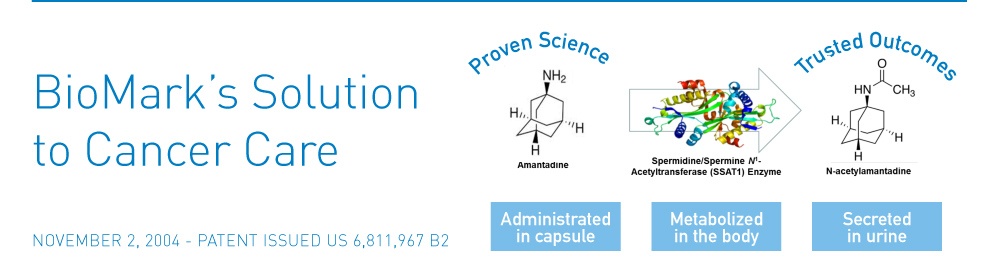
BioMark researches and screens the acetylated form of Amantadine. Amantadine, a drug given to cancer patients before measurement. LC MS in body fluids makes this screening possible.
An enzyme called Spermine/Spermidine N-Acetyl Transferase (SSAT) triggers the aceytylation. Studies show that many cancers display elevated levels of SSAT. These cancers include lung, breast, prostate, melanoma and GI cancers.
The principles of metabolomics are well proven in ongoing clinical trials. These trials test both healthy subjects and cancer patients. The analysis of SSAT mRNA tissue samples allows oncologists to identify cancer types.