Metabolomics
What is Metabolomics?
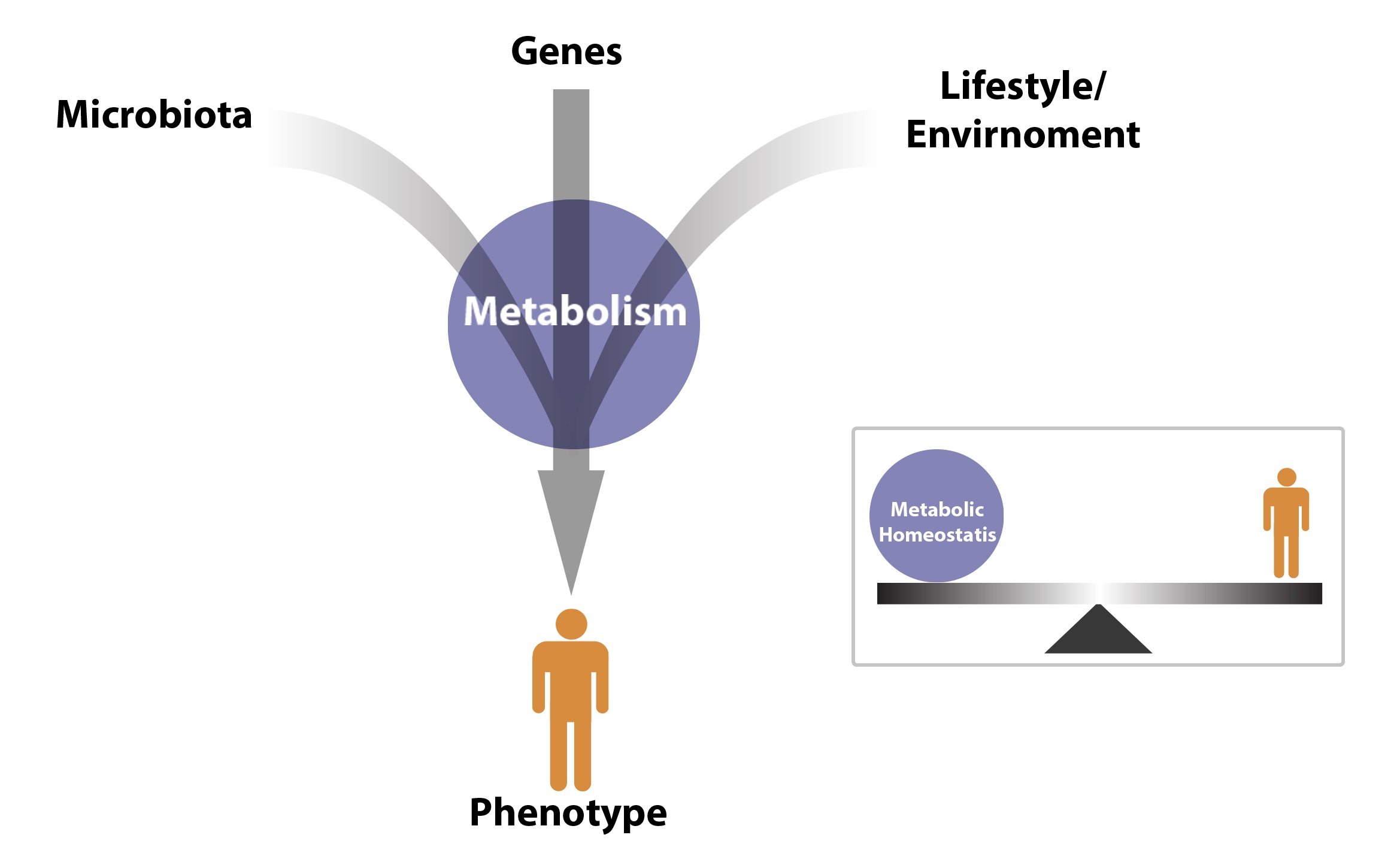
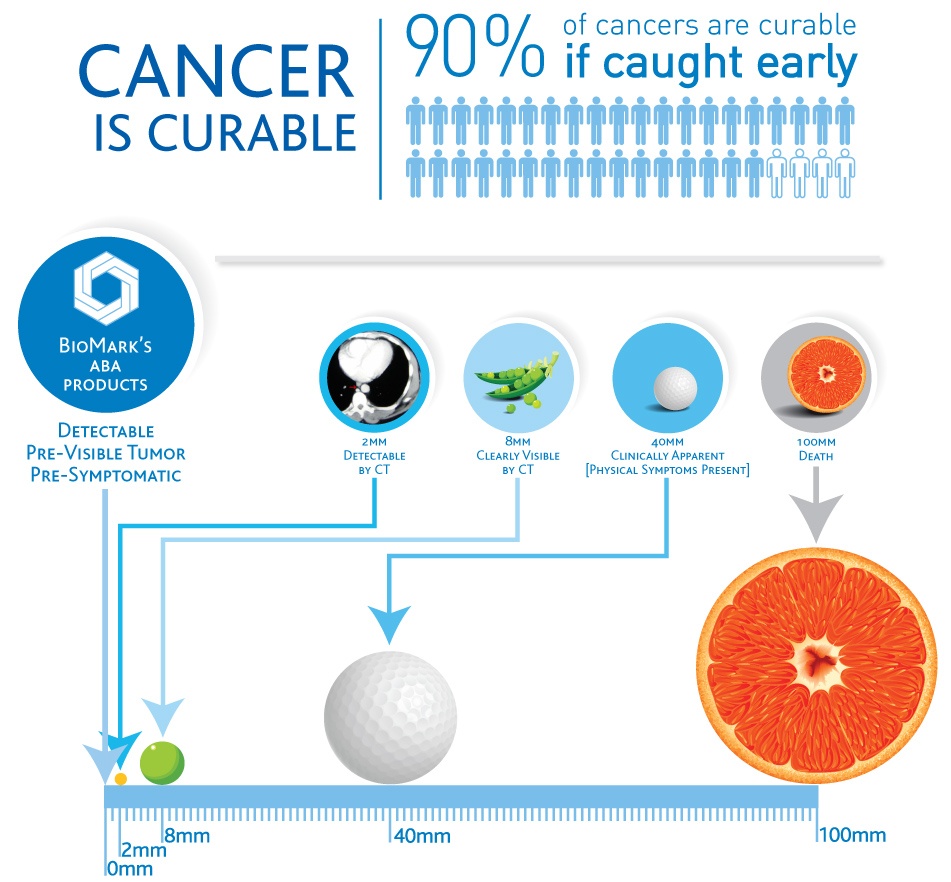
BioMark’s research and development is based on the science of metabolomics. Our studies focus on the pivotal role that metabolomics plays in early cancer detection with an aim to improve patient outcome.
Our research and technology development consist of:
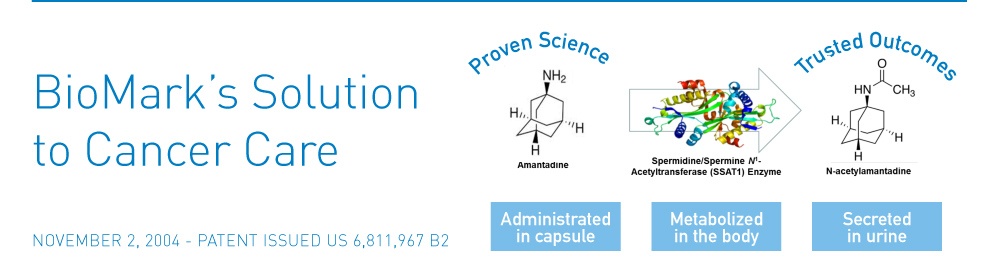
- Screening for the acetylated form of an FDA approved drug (called Amantadine) given to patients prior to measurement. This is done via LC MS in body fluids. This acetylation is performed by the enzyme called Spermine/Spermidine N-Acetyl Transferase (SSAT). It has been documented that elevated levels of SSAT are observed in many cancers including lung, breast, prostate, and GI cancers. Clinical trials on patients with cancer, as well as healthy subjects, provided proof of principle. In addition, studies in analysis of SSAT mRNA levels in tissue samples demonstrated elevated level of SSAT in various cancers. (See section under WHY)
- Use of high performance metabolites for tissue specific cancers (see section under – CAN WE DETECT SPECIFIC TYPES OF CANCERS USING METABOLOMICS?)