Biomark to Expand Treatment Response Trial to Advanced Lung Cancer Patient Receiving Immunotherapy

Vancouver, British Columbia – (June 29th, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce that Health Canada has approved its clinical trial application (CTA) and has granted a Letter of No Objection (NOL) for its application entitled Excretion of Acetylamantadine (AA) by Lung Cancer Patients During a […]
BIOMARK’S COLLABORATOR RECEIVES FUNDING TO VALIDATE ITS BIOMARKER PANEL FOR THE EARLY DETECTION OF LUNG CANCER
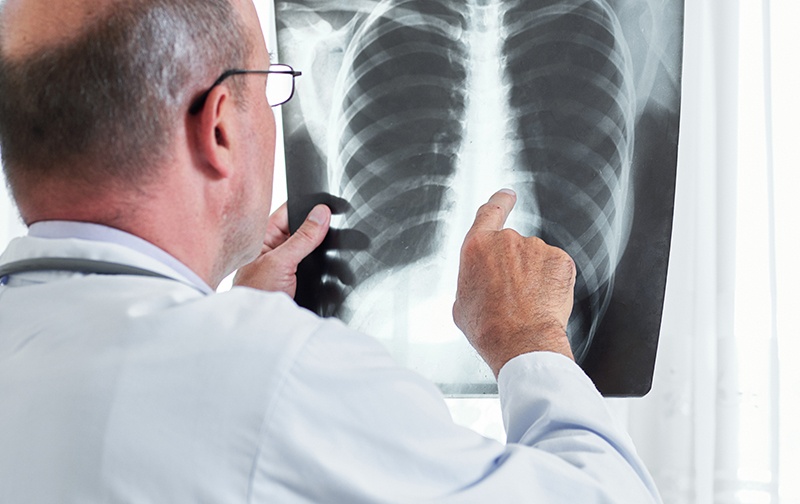
Vancouver, British Columbia – (March 16, 2021) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce today that its sponsored research collaboration with The Metabolomics Innovation Centre (TMIC) was successful in receiving funding from the Novel Technology Application in Cancer Prevention and Early Detection Spark Grants competition. This comes […]
BIOMARK TEAM INVITED FOR FULL APPLICATION FOR SPARKS GRANT COMPETITION

Vancouver, British Columbia – (September 14th, 2020) – BioMark Diagnostics Inc. (“BioMark”) (CSE: BUX) (FSE: 20B) (OTCMKTS: BMKDF) is pleased to announce that 2 of its most recent abstract submissions for the Novel Technology Application in Cancer Prevention and Early Detection Spark Grants competition have resulted in invitations to submit full applications for funding. This […]
